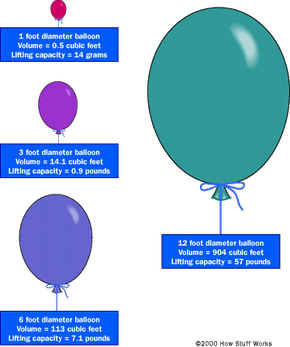
Build Your Own Hot-Air Balloon: Volume I - Design Criteria: Balloons, Eagle, Rechs, Robert J., de Piolenc, F. Marc: 9781519248909: Amazon.com: Books
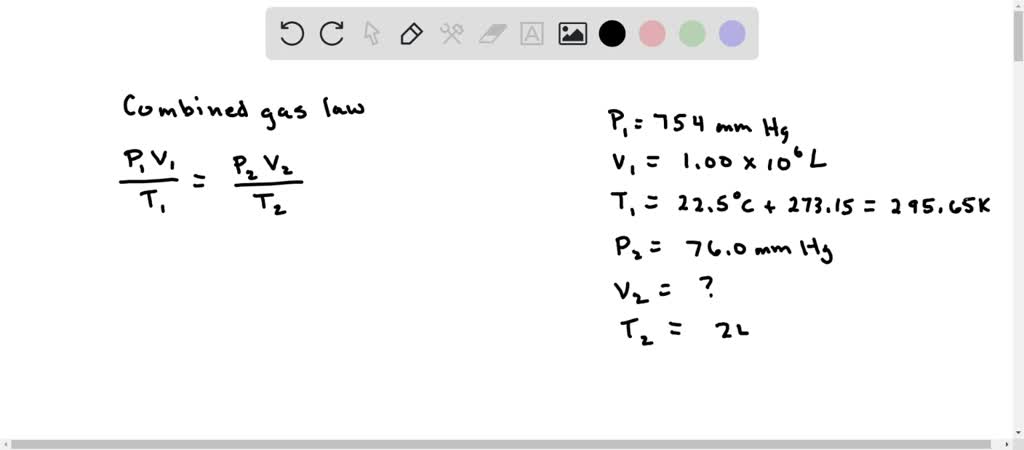
SOLVED: A helium balloon has a volume of 1.00 x 106 L at ground level with the pressure of 754 mm Hg and a temperature at 22.5 C. What is its volume

Question Video: Understanding How the Volume of a Balloon Changes as the Temperature Is Changed | Nagwa
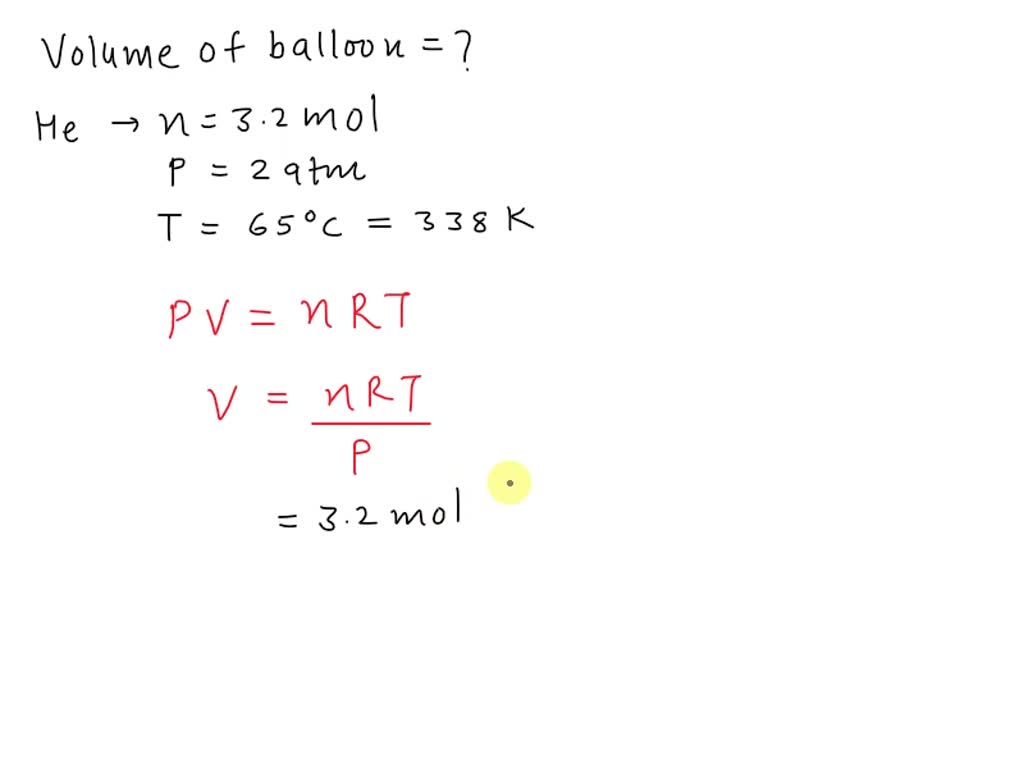
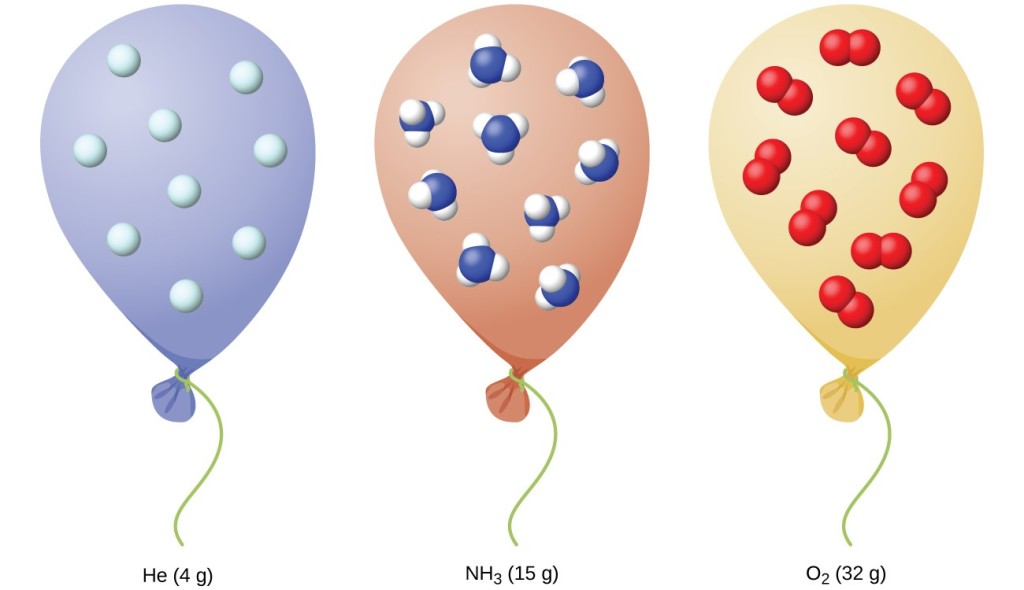
SOLVED: What is the volume of a balloon if contains 3.2 moles of helium at a temperature of 65 %C and pressure of 2.0 atmosphere?
A spherical balloon is increasing in volume at 24π cm^3/s How fast is the radius of the balloon increasing when the surface area of the balloon is 30π cm^2? - Quora

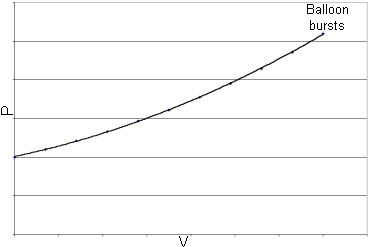
homework and exercises - The Official Equation for the relationship between the Volume of a balloon and time - Physics Stack Exchange

A balloon has a volume of 3.00 liters at 24.0°c. the balloon is heated to 48.0°c. calculate the new volume - brainly.com

SOLVED: A helium-filled balloon has a volume of 15.8 L at a pressure of 1.00 atm. What is the volume of the balloon high in the atmosphere where the pressure is 600.
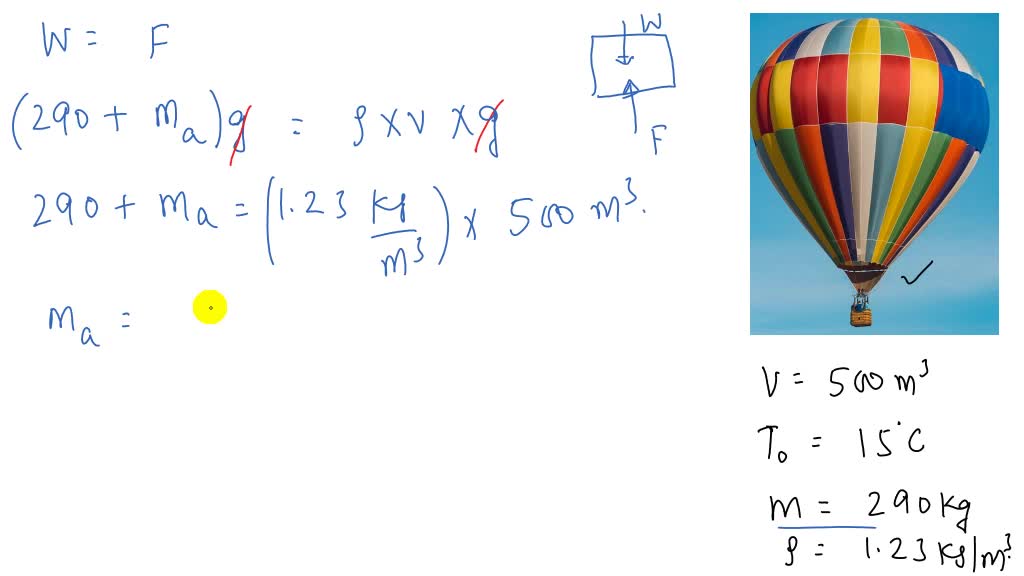
SOLVED:A hot-air balloon stays aloft because hot air at atmospheric pressure is less dense than cooler air at the same pressure. If the volume of the balloon is 500.0 m^3 and the



![Q. 8.12 A balloon is filled with helium ... [FREE SOLUTION] | Vaia Q. 8.12 A balloon is filled with helium ... [FREE SOLUTION] | Vaia](https://s3.eu-central-1.amazonaws.com/studysmarter-mediafiles/media/textbook-exercise-images/image_PYBt1mW.png?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA4OLDUDE42UZHAIET%2F20230813%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20230813T053720Z&X-Amz-Expires=90000&X-Amz-SignedHeaders=host&X-Amz-Signature=75489340dbb59274e828ae2ec7ad88311f299ef54df8c999f831e26d27c267fe)